LIMS Software for Lab Quality Control, QA, and QC Testing
Flexible QC LIMS Software for Your Lab’s Tests and QA Workflows
Core QC Parameters, QA Reports & Compliance for Laboratory Quality Control
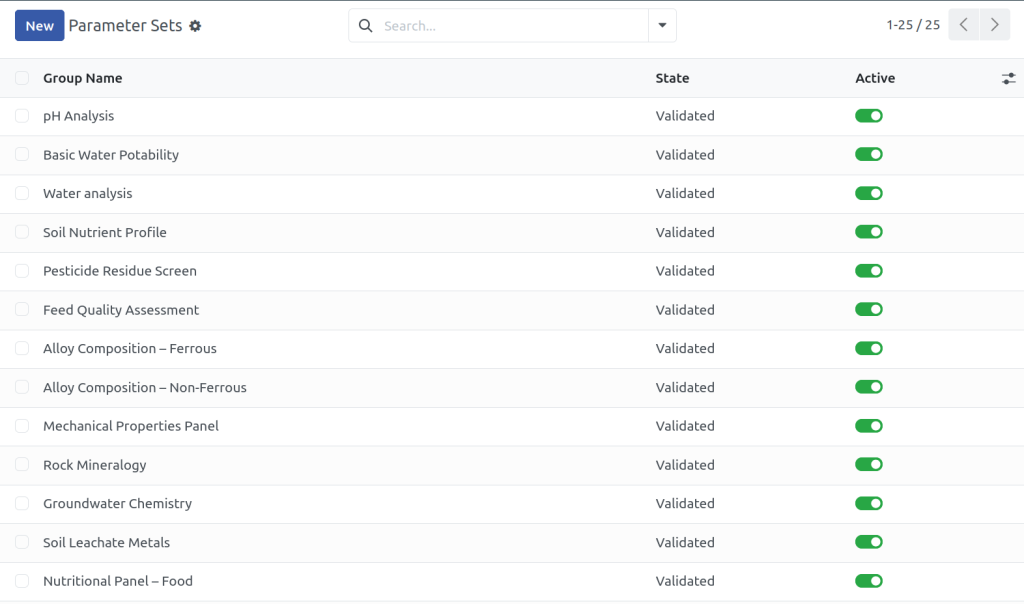
Common QC Parameters
pH
Conductivity
Moisture Content
Viscosity
Density
Ash Content
Tensile Strength
Melting Point
Color (Visual / Spectrophotometric)
Particle Size Distribution
Microbial Load
Contaminants (Pb, Hg, As, etc.)
Residual Solvents
Surface Roughness
Odor / Appearance
QA & QC Reports
Batch Quality Reports
Calibration Certificates
QC Summary Sheets
COA (Certificate of Analysis)
Internal Audit Reports
Non-Conformance Reports
Corrective Action Logs
Sample Receiving & Log Sheets
Process Validation Records
Trend & Deviation Analysis Charts
Test Method Validation
Product Release Forms
Raw Data Entry Sheets
Equipment Qualification Logs
…etc.
Compliance Standards
ISO/IEC 17025
ISO 9001
GMP / GLP Compliance
FDA 21 CFR Part 11
Internal SOP & QA Policies
Customer-Specific QA Requirements
Analyst Signature & Review Logs
Traceability & Chain of Custody
Method Validation Protocols
Audit Readiness Requirements
Retention & Record Control Policies
Confidentiality & Data Integrity
National / Industry-Specific Guidelines
…etc.
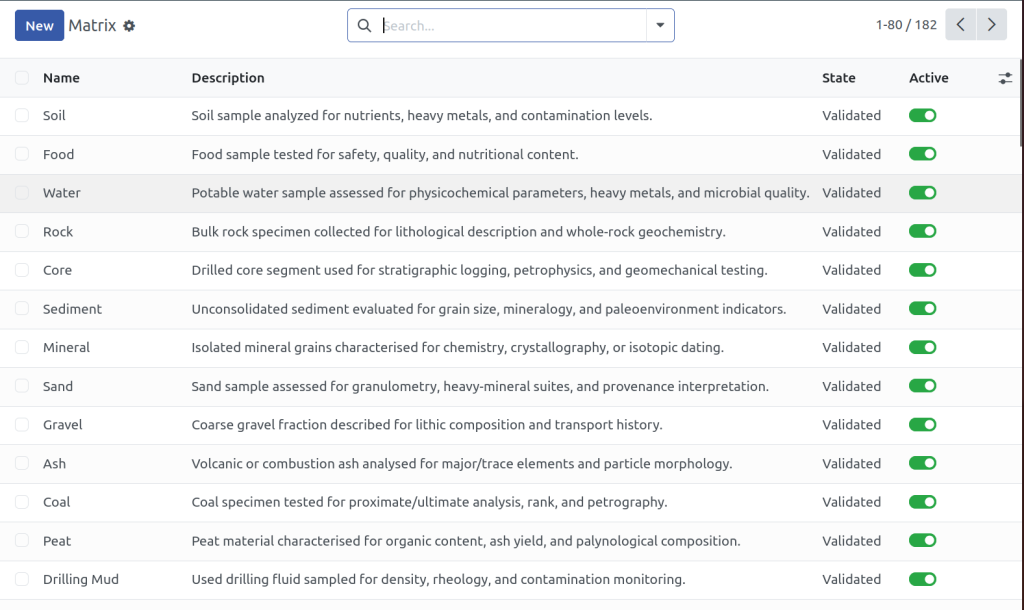
Key Features Built for Industrial QC & QA Labs
Our LIMS is purpose-built for quality control testing in laboratories. Whether you're managing pretreatment samples, auditing QA processes, or enforcing ISO standards, this QC LIMS gives you the tools to stay compliant and in control.

LIMS Software for QA, QC and Lab Quality Control, Built on Odoo
LIMS.SCIENCE runs entirely on Odoo, a powerful ERP system trusted by over 15 million users across the world.
This laboratory quality control software supports industrial labs with full QC workflows, test method validation, QA audits, and compliance to ISO 17025 or 9001.
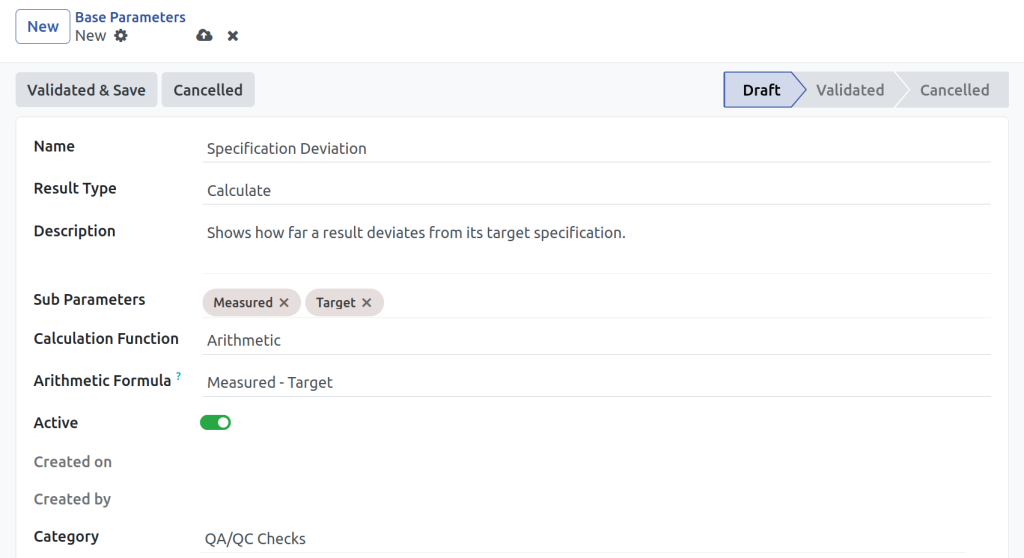
- Configure test methods, limits, and units for industrial QA and QC needs
- Track sample flow, storage conditions, and test timelines across lab stages
- Approve or reject results with full audit trails and deviation tracking
- Assign user roles so QA leads, lab staff, and clients access only what they need
- Manage multiple lab sites and processes from a single dashboard
- Access securely in the cloud or on your own servers
Need Help?
FAQs
Can I customize test methods and limits?
Yes. You can set your own test parameters, limits, and units for different products, materials, or workflows.
Does this LIMS support ISO 9001 or ISO 17025 compliance?
Yes. It includes tools to help you enforce SOPs, maintain audit trails, log analyst signatures, manage traceability, and generate standard-compliant reports.
Can multiple labs or plants use the same system?
Absolutely. The LIMS can be configured to manage multiple locations or departments in one centralized platform. Each site can have its own tests, users, and workflows, while management gets unified oversight through role-based dashboards and reports.
Does the system support non-conformance and corrective actions?
Yes. You can document out-of-spec results, classify non-conformances, assign corrective or preventive actions (CAPA), and track resolution status.
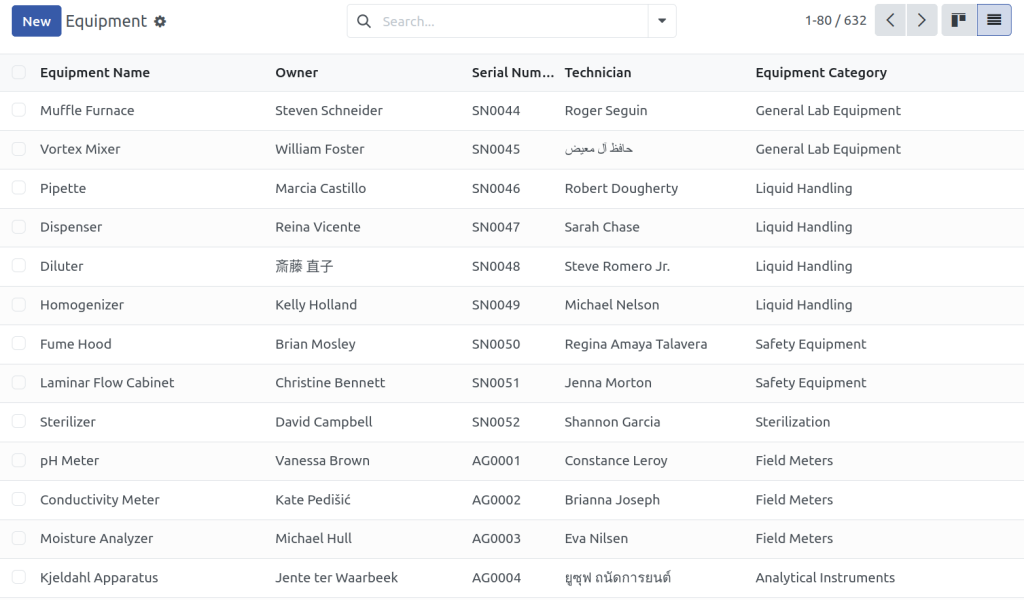
Can I manage instrument calibration and maintenance schedules?
Yes. You can log calibration certificates, schedule future calibrations, and track equipment maintenance history. Alerts can be configured to notify you of upcoming due dates, helping ensure your instruments are always audit-ready and within spec.
Can I export data or reports for audits?
Yes. You can generate and export reports in multiple formats (PDF, Excel, CSV), making audit prep quick and hassle-free.
Make Quality Control Easier to Manage
Handle lab audits, QC reports, pretreatment tests, and compliance in one LIMS platform designed for quality control.