Genomics LIMS for Next-Generation Sequencing Labs
Built for Genomics Complexity
Core Genomics Entities
Samples and specimen types
DNA/RNA extractions
Library preparations
Library QC measurements
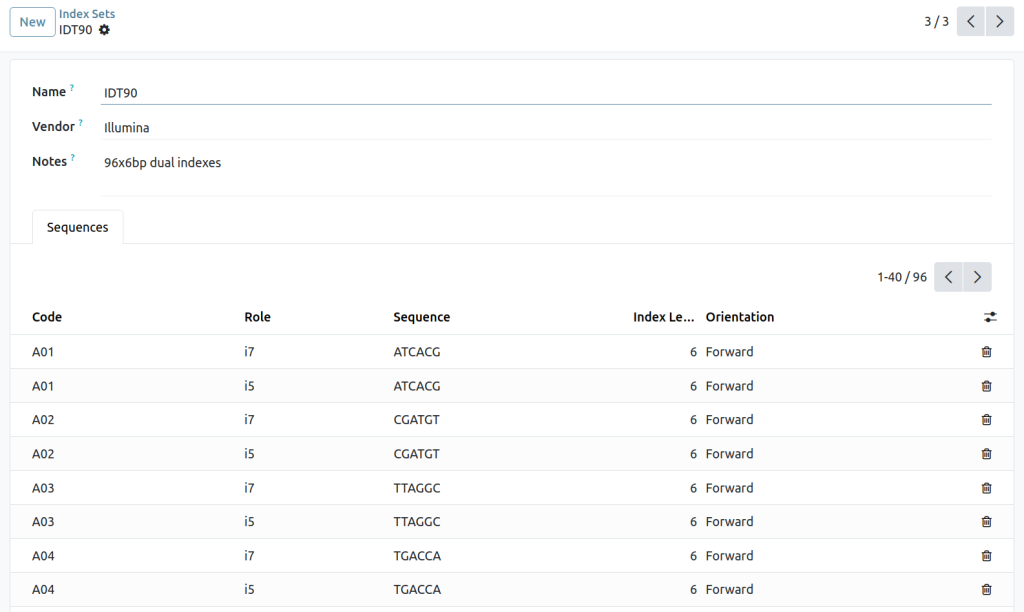
Index assignments
Pooled libraries
Pool quantification
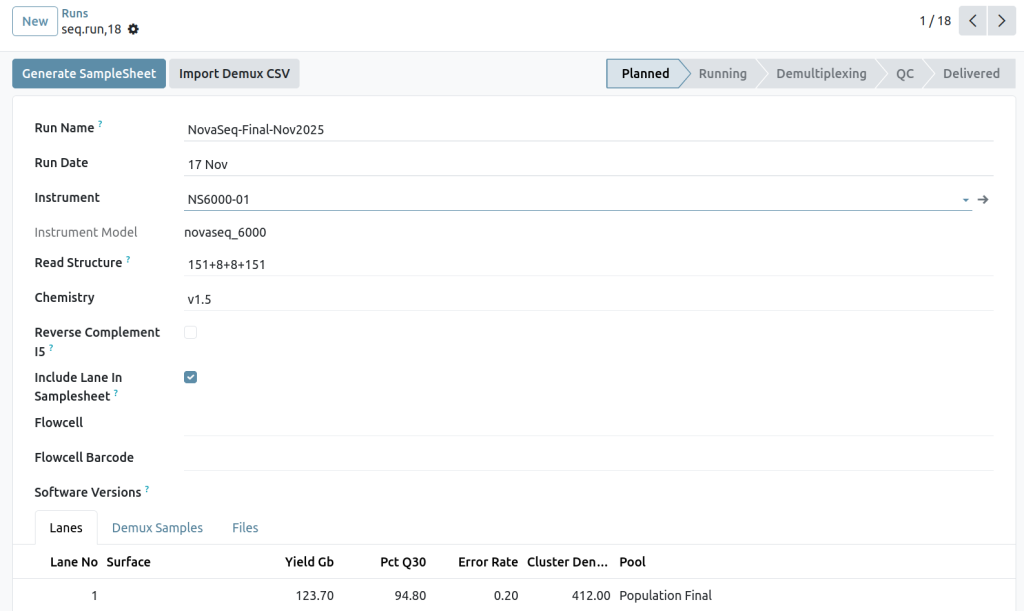
Sequencing runs
Lane assignments
Read structures
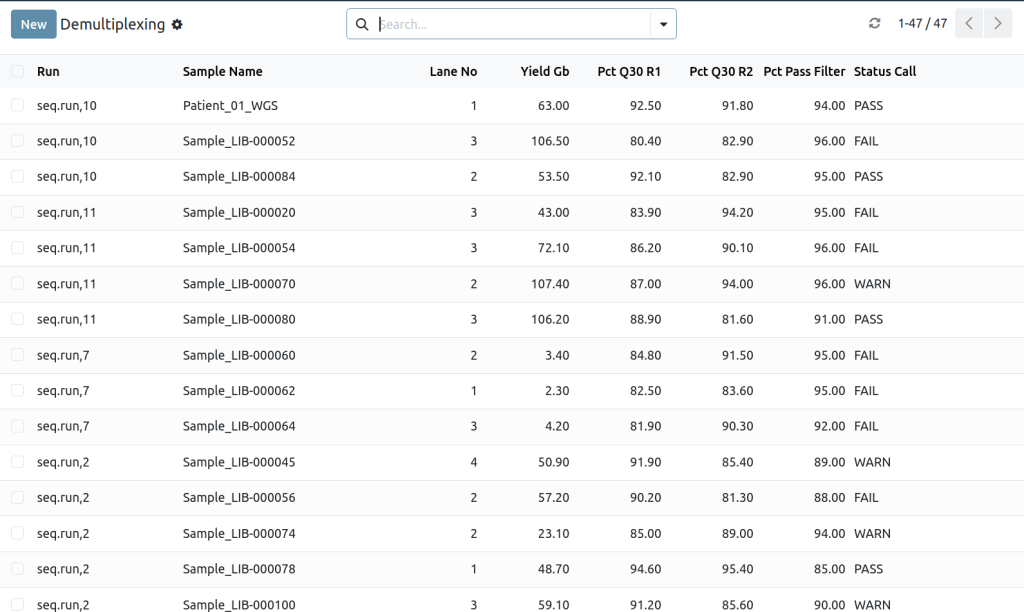
Demultiplexed samples
Fastq file tracking
Analysis pipeline runs
Quality control checkpoints
Projects and studies
Customer deliverables
Reagent lot tracking
Index sets and barcodes
Sequencing instruments
Storage locations
Integration Capabilities
Illumina NovaSeq systems
Illumina NextSeq platforms
Illumina MiSeq instruments
Oxford Nanopore MinION
Oxford Nanopore PromethION
qPCR quantification systems
Bioanalyzer integration
TapeStation data import
Fragment Analyzer results
Qubit fluorometer measurements
Plate readers
Thermocyclers
LIMS-to-bioinformatics pipelines
Sample sheet generation
Automated demux CSV import
BaseSpace integration
Custom API connections
Laboratory automation systems
Barcode scanners
Compliance & Traceability
ISO 17025 compliance
ISO 15189 compliance
Complete audit trails
Chain of custody tracking
Sample lineage tracking
Version control
User access controls
Role-based permissions
Electronic signatures
Instrument calibration records
Equipment maintenance logs
Method validation documentation
Standard operating procedures
Quality management integration
Non-conformance tracking
Corrective action records
Regulatory reporting
Data integrity controls
Batch record management
Document management
Training records
Key Features for Next-Generation Sequencing Labs
Purpose-built capabilities that address the unique complexity of genomics workflows, from preventing index clashes to ensuring regulatory compliance.

Genomics Laboratory Management System Built on Odoo
LIMS.SCIENCE runs entirely on Odoo, a powerful ERP system trusted by over 15 million users across the world.
We've adapted it specifically for next-generation sequencing laboratories. You can manage everything from sample tracking and library preparation to sequencing runs, demultiplexing, and compliance in one place.
- Configure sequencing protocols, index sets, and QC thresholds for your specific NGS workflows
- Track samples through library prep, pooling, sequencing runs, and demultiplexing with complete traceability
- Approve or reject results with full audit trails, including QC checks, run validation, and quality flags
- Assign user access by role so lab techs, bioinformaticians, QC managers, and clients see only what they need
- Centralize multi-site sequencing operations across research facilities, clinical labs, or service providers in one dashboard
- Access anywhere via the cloud or install on your own secure server
Need Help?
FAQs
Is this LIMS compliant with ISO 17025 for clinical sequencing labs?
Can the system handle multiple sequencing platforms and instruments?
How does the LIMS prevent index clashes in multiplexed runs?
Can we customize workflows for our specific sequencing protocols?
What sample types and sequencing applications are supported?
How does demultiplexing data get into the LIMS?
Start Managing Your Sequencing Operations with Purpose-Built Genomics LIMS
Experience how specialized laboratory management software can streamline your NGS workflows from sample tracking through data delivery. See the system in action with a personalized demo tailored to your sequencing operations.