Picture this scenario. An auditor walks into your lab and asks to see the raw chromatography data behind a result you reported six months ago. Your QA manager starts sweating. The analyst who ran the test has left the company. The file is somewhere on a shared drive, maybe in a folder called “OLD DATA 2023,” or was it on the instrument PC that got reformatted last month?
This is the reality of modern laboratory data management. Labs generate more data than ever before, yet most of that data lives scattered across instrument hard drives, USB sticks, email attachments, and network folders with naming conventions that only made sense to whoever created them.
The problem is not a lack of data. The problem is finding it, proving it has not been tampered with, and connecting it back to the sample it belongs to.
This is where LIMS and SDMS enter the conversation. These two systems are often confused, sometimes sold as interchangeable, and frequently misunderstood. But they solve fundamentally different problems.
Think of it this way: LIMS is the traffic controller that directs your samples through the testing process. SDMS is the library archive that preserves every piece of raw evidence those tests generate.
Understanding this distinction determines whether you spend money on the right system, whether your audits pass without drama, and whether your valuable research data survives the next server migration.
SDMS vs LIMS: The Differences
Before we go deeper, here is a quick overview of differences between a LIMS and an SDMS:
| Feature | LIMS | SDMS |
|---|---|---|
| Primary Focus | The physical sample and its journey | The digital file and its preservation |
| Data Type | Structured (results, metadata, specifications) | Unstructured (raw files, spectra, images) |
| Compliance Role | Process control and audit trails for workflows | Data integrity and original record preservation |
| Typical User | Lab technicians, QC managers, sample coordinators | IT administrators, data managers, compliance officers |
| Key Output | Certificates of Analysis, test reports | Searchable archive of raw instrument data |
| Core Question | What happened to this sample? | Where is the proof? |
What is a LIMS?
A Laboratory Information Management System exists to answer one question: what is happening to this sample right now, and what needs to happen next?
When a sample arrives at your lab, it needs to be logged, labeled, assigned to analysts, routed through the correct tests, have its results recorded, checked against specifications, and ultimately reported. Without a system to manage this, you end up with spreadsheets, sticky notes, and the constant anxiety of wondering whether something fell through the cracks.
A modern LIMS handles:
- Sample registration and tracking. Every sample gets a unique identifier. You know where it is, who has it, and what stage it has reached.
- Workflow management. Tests are assigned to analysts based on qualifications, workload, and equipment availability.
- Specification checking. Results are automatically compared against defined limits. Pass or fail is determined without human interpretation.
- Report generation. Certificates of Analysis (CoAs) are produced automatically with all required data in the correct format.
- Audit trails for process compliance. Every action is logged. Who did what, when, and why.
What is an SDMS?
A Scientific Data Management System solves the problem LIMS was not built to solve: preserving and organizing raw instrument data in a way that meets regulatory requirements and remains accessible for years.
SDMS is often called the “invisible hero” of laboratory informatics. Users rarely interact with it directly. It runs in the background, automatically sweeping files from instrument PCs, tagging them with metadata, and storing them in a secure, searchable archive.
The key functions of an SDMS include:
- Automated file capture. No more USB sticks or manual file transfers. Files move from instrument to archive automatically the moment they are generated.
- Metadata tagging. Raw files are linked to sample IDs, project names, analysts, and dates. Metadata is the structured information that describes unstructured files, making them searchable.
- Universal viewing. This solves a painful problem. Without SDMS, you need the original instrument software to view a data file. If that instrument is retired or the software license expires, your historical data becomes inaccessible. SDMS typically includes viewers that let you open files without the original software.
- Data integrity enforcement. This is where ALCOA+ comes in. ALCOA+ is a regulatory framework requiring that data be Attributable, Legible, Contemporaneous, Original, and Accurate, plus complete, consistent, enduring, and available. SDMS preserves the original record with timestamps and access controls that prove the data has not been modified.
The importance of “Original” in ALCOA+ cannot be overstated. Regulators like the FDA do not just want to see your reported result. They want to trace that result back to the original instrument output. If you cannot produce that original file, or if there is any question about whether it has been altered, you have a data integrity problem.
How LIMS & SDMS Work Together
Understanding how LIMS and SDMS work together is best illustrated by following a sample through a complete testing cycle.
- Step 1: Sample registration. A water sample arrives at the lab. The sample coordinator logs it into LIMS, which generates a unique sample ID (let’s say WTR-2026-0847) and assigns the required tests based on the customer’s order.
- Step 2: Test execution. An analyst runs the sample on an ICP-MS instrument for heavy metals analysis. The instrument generates a raw data file containing all the spectral data.
- Step 3: Automatic file capture. The moment the run completes, SDMS detects the new file on the instrument PC. It automatically captures the file, timestamps it, and tags it with the sample ID that the analyst entered before the run.
- Step 4: Result transfer. The analyst reviews the data and approves the calculated result. LIMS receives the final value (for example, Lead: 0.005 mg/L) either through direct instrument integration or manual entry.
- Step 5: Linking and reporting. The LIMS record for WTR-2026-0847 now shows the result and includes a hyperlink to the raw file stored in SDMS. When the Certificate of Analysis is generated, an auditor can click through from the reported value to view the original instrument output.
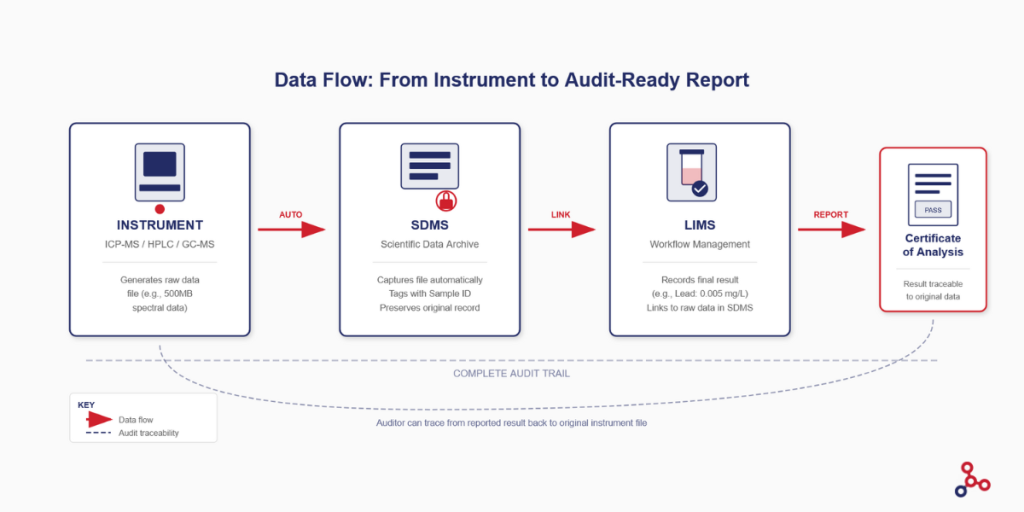
This integration means that the result and its proof are permanently connected. Six months or six years later, you can reproduce the complete picture: what the result was, who reported it, and the raw data that supports it.
LIMS vs SDMS: Which One Do You Need?
Your decision depends on your lab type, regulatory environment, and pain points.
Scenario A: The Manufacturing or QC Lab
Profile: High sample volume, routine tests, focus on throughput and turnaround time.
Priority: LIMS. Your biggest challenge is managing the flow of samples and getting results out the door efficiently. A good LIMS will dramatically reduce manual data entry, prevent samples from getting lost in the queue, and ensure every result is checked against specifications before release. SDMS becomes important when you have regulatory inspections that require raw data access, but many QC labs manage with network storage for instrument files initially.
Scenario B: The R&D or Contract Research Lab
Profile: Complex experiments, non-routine methods, high intellectual property value.
Priority: SDMS may be the first investment. Your raw data is your product. The experiments you run today could become the foundation of a patent filing or regulatory submission years from now. Losing that data, or being unable to prove its integrity, could be catastrophic. An SDMS protects this IP by creating an immutable record of all experimental data with full audit trails.
Scenario C: The Regulated Lab (FDA, ISO 17025, GLP)
Profile: Subject to FDA 21 CFR Part 11 (the regulation governing electronic records and signatures), ISO 17025 accreditation, or Good Laboratory Practice requirements.
Verdict: You need both. Regulatory frameworks increasingly require complete traceability from reported result to original data. 21 CFR Part 11 specifically requires that electronic records be protected against modification, have complete audit trails, and remain attributable to specific individuals. Achieving this with LIMS alone is difficult because LIMS was not designed to be a file archive. Achieving it with SDMS alone is incomplete because SDMS does not manage sample workflows. The combination gives you defensible compliance.
FAQ and Common Misconceptions
How is SDMS different from LIMS?
LIMS manages samples and workflows. It tracks physical samples through the testing process and records structured results. SDMS manages files and archives. It captures raw instrument data and preserves it with metadata that makes it searchable and compliant. LIMS tells you what the result was. SDMS stores the evidence that proves it.
Can I just use a shared network drive or SharePoint instead of SDMS?
For compliance purposes, no. Network drives and document management systems like SharePoint lack the critical audit trail capabilities required by regulations. They do not automatically capture files from instruments.
They do not prevent files from being modified or deleted. They do not prove that the file you have today is identical to the file that was created during the original test. They do not link files to sample IDs in a queryable way. For informal data storage in a non-regulated environment, a network drive can work. For any lab facing audits or compliance requirements, it creates significant risk.
What is the typical cost difference between LIMS and SDMS?
Costs vary widely based on scale and features, but LIMS implementations typically represent the larger investment because of their complexity and customization requirements. SDMS costs are often driven more by storage infrastructure and the number of instruments being captured. Some organizations spend more on SDMS when they have high data volumes and complex instrument fleets. The key is to evaluate based on your specific needs rather than assuming one category is always more expensive.
Audit Your Data Integrity Gaps
If this article has made you uncomfortable about the state of your laboratory data, that discomfort is valuable. Most labs operate with significant data integrity gaps that only become apparent during audits, regulatory inspections, or when critical data cannot be located.
At LIMS.Science, we help laboratories implement information management systems that solve real operational problems while meeting compliance requirements. Our solutions are built on proven ERP architecture, offering the workflow management capabilities of a full LIMS with the flexibility to integrate with specialized data management tools as your needs grow.
Whether you need to start with sample management fundamentals or build a complete LIMS-SDMS integration, we can help you design an approach that fits your laboratory type, budget, and regulatory environment.
Book a demo to discuss your laboratory’s specific challenges and explore how modern informatics can eliminate the data chaos. Visit lims.science to schedule a demo or contact our team directly.

